- Home
- Academics
- Undergraduate Research
- Celebrating Undergraduate Research
- Daniel Heintzelman
Daniel Heintzelman
Class of 2021
- Chemistry

Kinetic Investigations of NOx Chemistry Pertaining to the Global Nitrogen Cycle
Project Mentor:
- Anthony Rizzuto, assistant professor of chemistry
Project Abstract
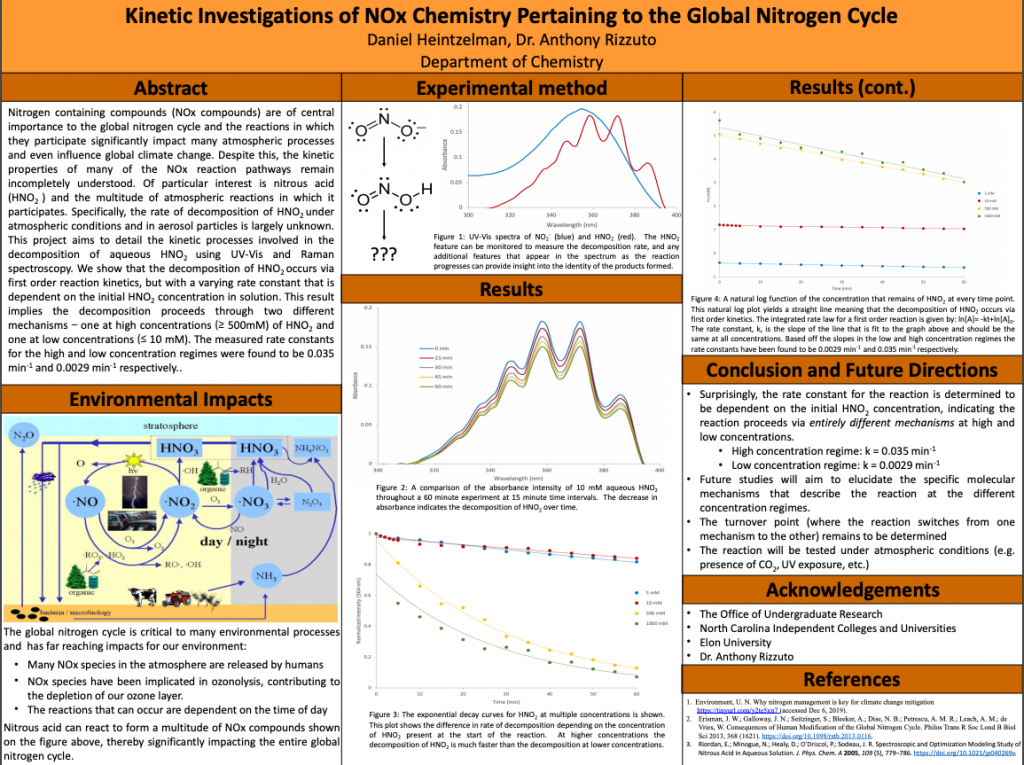
Nitrogen containing compounds (NOx compounds) are of central importance to the global nitrogen cycle and the reactions in which they participate significantly impact many atmospheric processes and even influence global climate change. Despite this, the kinetic properties of many of the NOx reaction pathways remain incompletely understood. Of particular interest is nitrous acid (HNO2) and the multitude of atmospheric reactions in which it participates. Specifically, the rate of decomposition of HNO2 under atmospheric conditions and in aerosol particles is largely unknown. This project aims to detail the kinetic processes involved in the decomposition of aqueous HNO2 using UV-Vis and Raman spectroscopy. We show that the decomposition of HNO2 occurs via first order reaction kinetics, but with a varying rate constant that is dependent on the initial HNO2 concentration in solution. This result implies the decomposition proceeds through two different mechanisms – one at high concentrations (³ 500mM) of HNO2 and one at low concentrations (£ 50 mM). The measured rate constants for the high and low concentration regimes were found to be 0.035 s-1 and 0.0029 s-1 respectively. Future studies will be conducted to elucidate the specific molecular mechanisms by which nitrous acid decomposes in the two concentration regimes.
 Download Project Presentation
Download Project Presentation